The cumulative effect of macromolecular crowding and surface adsorption on protein fibrillation
NIH, NIDDK, LBG
Travis Hoppe, Allen Minton, Di Wu
https://github.com/thoppe/Presentation_NIST_crowding
Biochemistry in the lab setting
Biochemistry in a
crowded environment
crowded environment


Biochemistry in a
crowded environment
(as an analytical model)
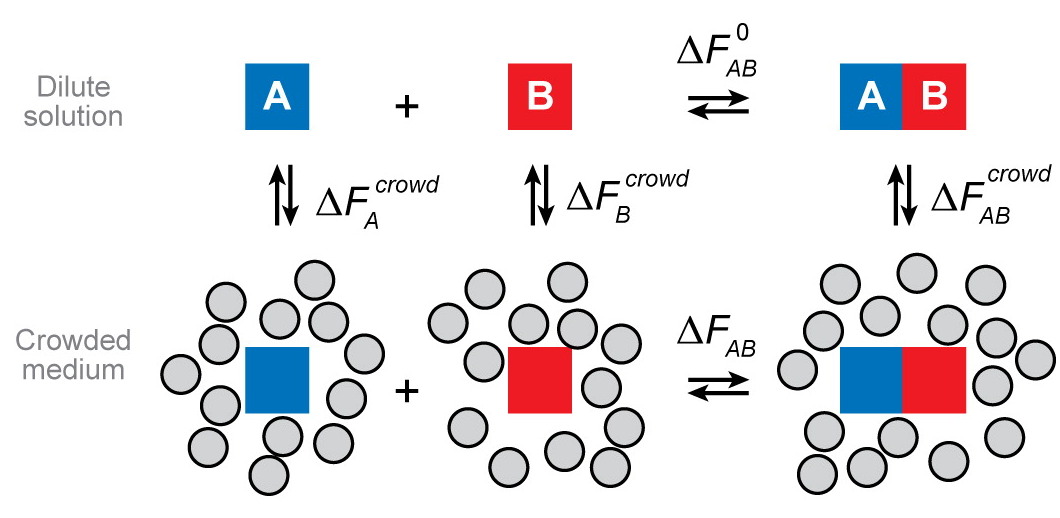
Background interactions: Weak, nonspecific interactions between macromolecular species and local constituents. Does not depends on detailed structure of interaction but only on gross physical properties like size, shape, hydrophobicity, net charge, dipole moment, ...
- What are the dominant interactions?
- What length scales are important?
- Dynamic or equilibrium?
- Experimental scope?
What is crowding?
(usually) repulsive nonspecific interactions with other soluble macromolecules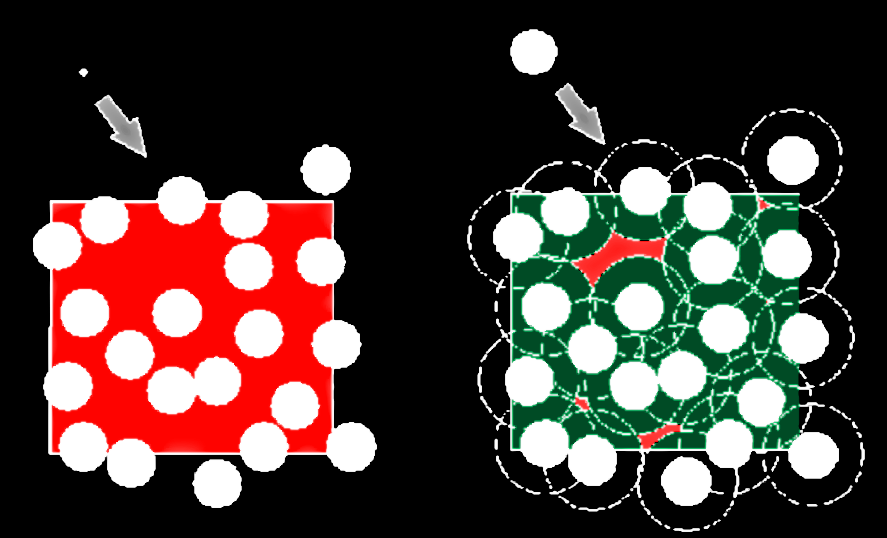
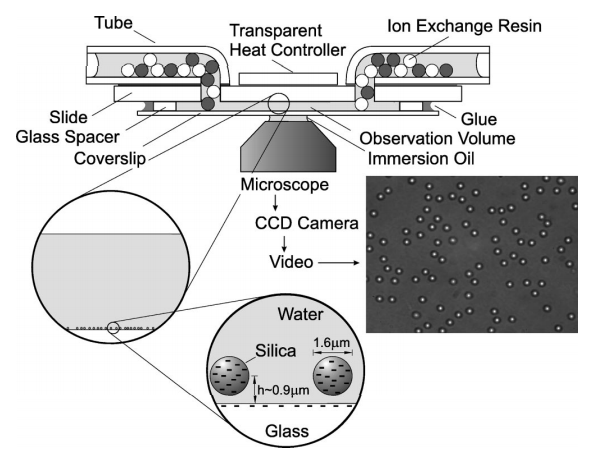
Experimental Evidence
Direct observation of the enhancement of non-cooperative proteinself-assembly by macromolecular crowding
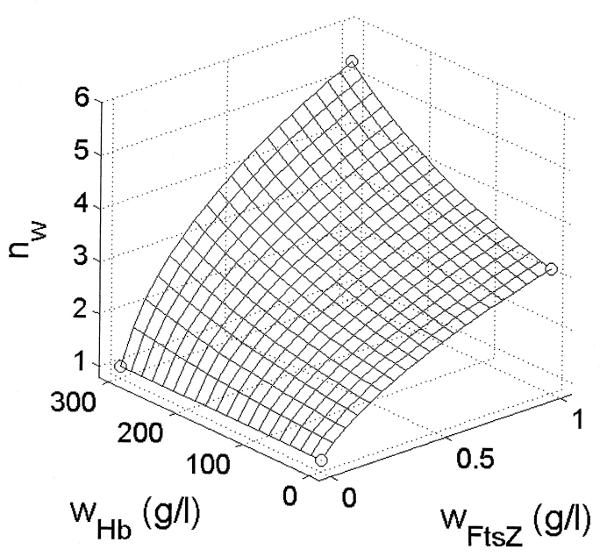
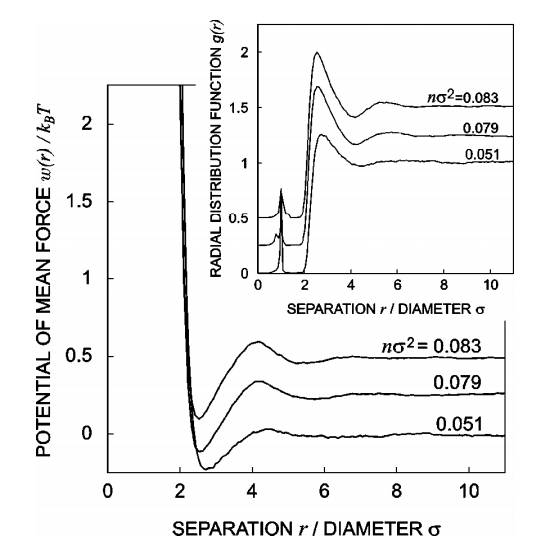
Experimental Evidence
Macromolecular Crowding Accelerates Amyloid Formationof apoC-II as a function of Dextran T10 concentration
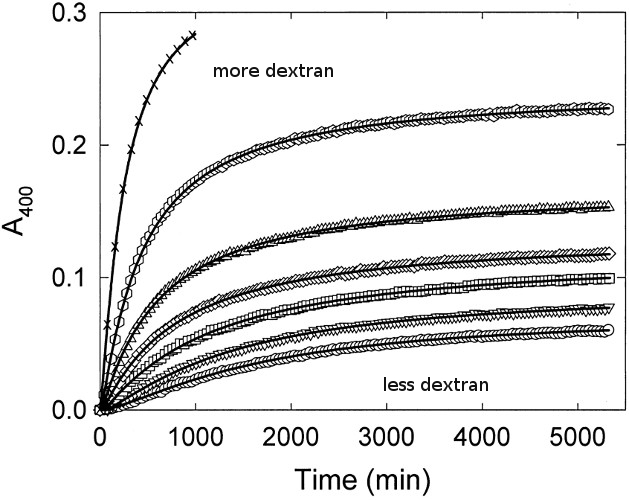
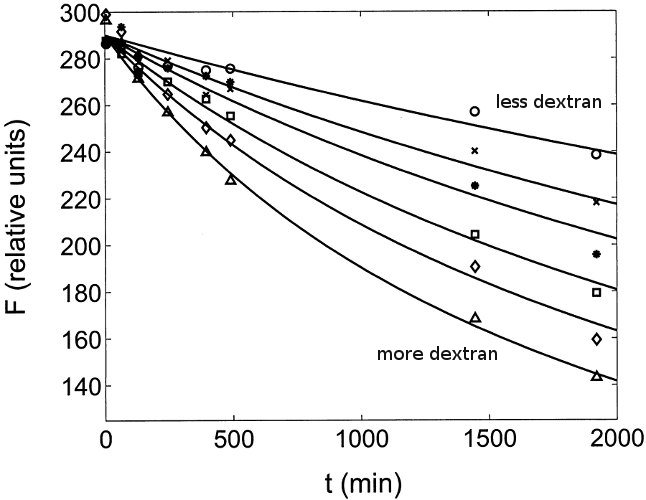
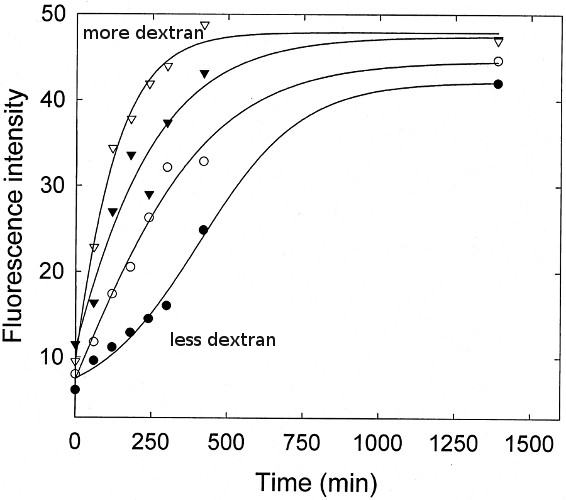
Experimental Evidence
Accelerated α-synuclein fibrillation from crowding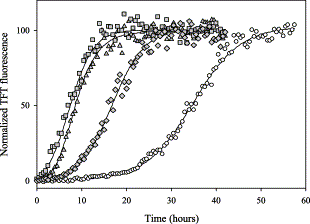
Model outline
restrict the domain- Fiber formation is isodesmic & linear
- Dilute macromolecular concentration
- Steric crowding interactions
- Spherical and sphereocylinder geometries
- Linear, isotropic adsorption
- Thermodynamic equilibrium
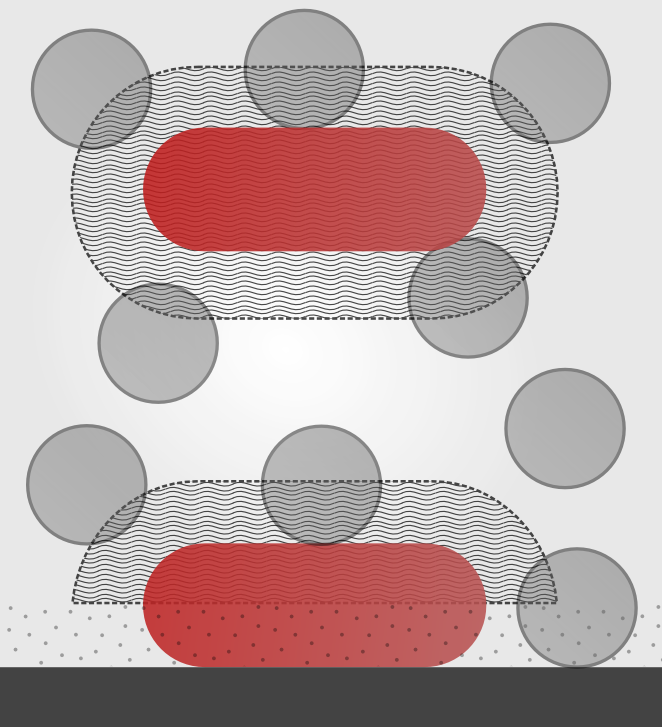
Model schematic
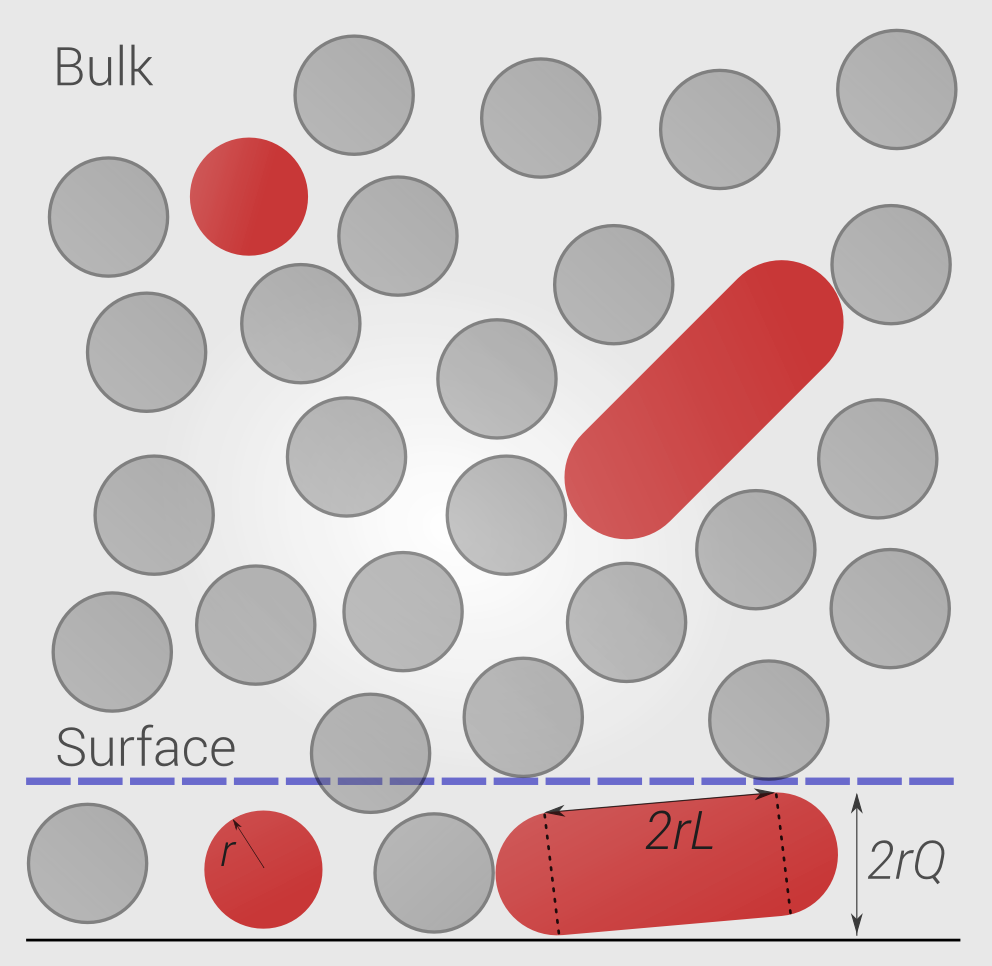
Partitioning between
bulk (b) and surface (s) compartments
Three terms: change of chemical potential & enthalpy/entropy of absorption
change in enthalpy during adsorption per monomer, and change in entropy upon adsorption.
Isodesmic fiber formation
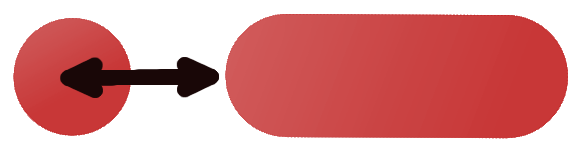
change in enthalpy during adsorption per monomer, and change in entropy upon adsorption.
Scaled particle theory
Thermodynamic cost of cavity formation
Hard-sphere case: Integrand can be evaluated exactly for close distances,
and approximated with the Percus-Yevick closure
of the Orstein-Zernike equation.
Reiss, Frisch, and Lebowitz, J. Chem. Phys. 31, 369 (1959)
Scaled particle theory
Can be extended to non-spherical geometries:Sphereocylinder into hard-spheres
Adsorption
Loss of entropy upon adsorption
Free rotation in bulk
Free rotation near the surface
is the width of the bounding surface.
Activity coefficient
two-body approximation
Activity coefficients depend only on excluded volume
Activity coefficient

Activity coefficient
multi-body approximation
is not constant near the surface (boundary effects)dependent on oligomer too (solute effects)
Effects can be real and measurable!
Activity coefficient
multi-body approximation
Use Monte-Carlo to estimate , i.e. the
dependence of crowding concentration.
Sample conformational states of hard-spheres near a boundary.
Two approaches to improve sampling, DMD (Discrete molecular dynamics) and particle displacement.
Widom sampling
Split into , sample over "test" particle:
Widom sampling (hard-spheres)
For hard-potentials and .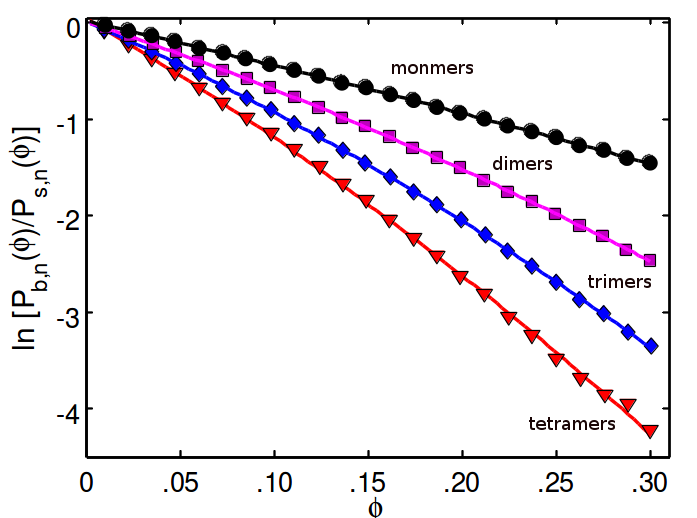
(monomers, dimers, trimers, and tetramers from black to red)
Linear dependence of on
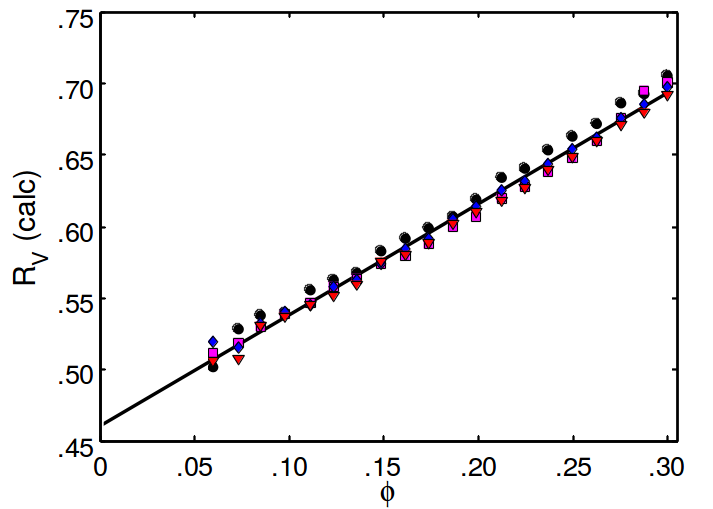
Results
Critical crowding concentration leads to unbounded oligomergrowth on the surface ... even in the absence of surface attraction!
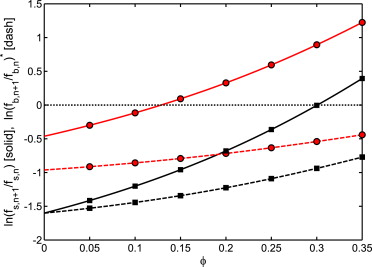
Red, ; Black .
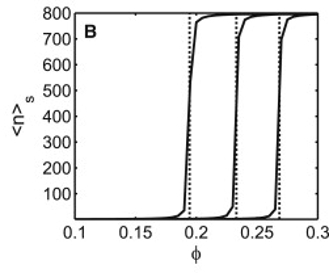
Left to right .
Qualitative predictions
Model predicts a critical "falling-out" of fiber growth in the solution at moderate, neutral and even repulsive surfaces.
Location of critical phenomena and extent of cooperatively is
sensitive to details of the model (adsorption/association).
Biological relevance?
The presence of large fibers in solution is unfavorable thermodynamically.
If fibrillation and/or adsorption is linked to biological function,
crowding can act as a sensitive form of biochemical regulation.
Thanks, you.
Laboratory of Biochemistry and Genetics
Physical Biochemistry Section


